http://www.theguardian.com/lifeandstyle/2015/jun/13/condom-testing-sex-science-gates-foundation#b06g22t20w15
It’s hugely unpopular, worn by an estimated 5% of men around the world: can 11 design mavericks, funded by Bill and Melinda Gates, reinvent the condom for a sexier, safer world?
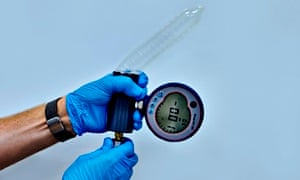 Testing times for condoms at a research facility in San Diego. Photograph: Steve Schofield for the Guardian
Testing times for condoms at a research facility in San Diego. Photograph: Steve Schofield for the Guardian
Brendan Weinhold and Christina Pederson began experimenting with new kinds of condoms four years ago, when they saw an ad on
Craigslist for couples to test contraceptives for cold hard cash.
Get paid to have sex? How could you say no?
“If anything was difficult, it was having to schedule sex,” Brendan says. “You know: ‘Ugh, we have to do it now.’ Filling in a calendar was bothersome.”
After sex, they had to fill in paperwork, recording all sorts of details for the
California Family Health Council (CFHC), America’s top testing facility for experimental condoms: whether they were both sober, which partner put the condom on, what positions they took, if the condom made any noise, if pubic hair snagged, how long they lasted, if either had an orgasm (and how many), if anyone felt pain. Plus the obvious: how far the condom came down the penis. “It was a bit strange writing down the amount of time we had sex for. ‘Are we good at having sex or bad at having sex?’” Christina says. Was the paperwork a mood-killer? “Somewhat, but it’s not the worst mood-killer we’ve encountered,” Brendan says.
Christina and Brendan, both 33, live in a house share in LA: she is an as-yet unpublished novelist, he is trying to make it as an actor. Christina had previously volunteered as a subject in a medical study that required her to lie in a hospital bed, donating blood, receiving intravenous drugs. “That made me feel like much more of a lab rat,” she says. “This time I felt as if I was actually doing something. It felt as if our opinions actually mattered.”
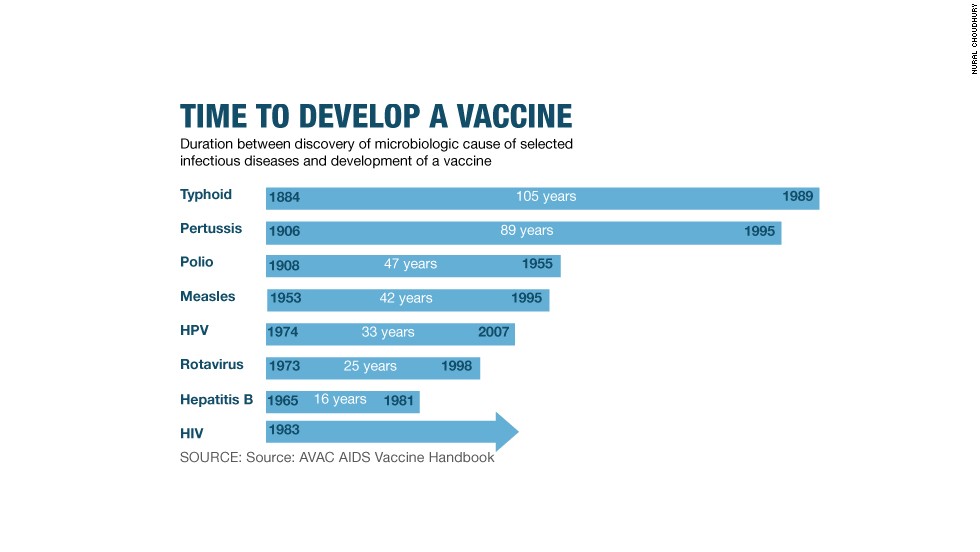
Brendan and Christina are part of an accelerating race for better 21st-century contraception, perhaps among the closest to understanding what future safe sex might look and feel like. The bottom line is, there are problems with the condom: while it is 98% effective when used correctly, it is hugely unpopular – worn by an estimated 5% of men worldwide. This is a fatal flaw in developing countries, where an HIV diagnosis remains a death sentence, due to the high cost of antiretroviral drugs. Condom design, almost unchanged in 120 years, is ripe for a makeover.
In 2013, Bill and Melinda Gates announced that
their Foundation was making condom innovation a priority (alongside toilets, vaccines and neonatal care): they offered a $100,000 grant to any team with a strong proposal for a “
next generation condom that significantly preserves or enhances pleasure, in order to improve uptake and regular use”.
The Foundation received more than 800 submissions, which in 2013 they narrowed down to
11 winners, announcing a further 11 winners of the grant in 2014. The successful proposals ranged from those using Nobel prizewinning materials (
graphene) to those with built-in applicators or lubricant. The Gates Foundation also gave money to simple behavioural studies, and to a shrink-to-fit condom dreamed up by the CFHC. Those proposals are now able to apply for phase two funding of up to $1m each – the winners will be announced later this year, with only a handful likely to be successful.
Meanwhile many of them are in the testing phase. To be enrolled in an official “breakage and slippage” study for the CFHC, you need to have been in a monogamous relationship for at least six months. The woman also needs to be on the pill or have an IUD, to ensure she won’t become pregnant. (Unless it’s an “efficacy” study, in which case you have to be monogamous but happy to accept the chance of getting a baby out of the study instead of a $100 cheque.)
Couples have sex for the CFHC between three and eight times with the experimental condom, then the same number of times with a placebo (a regular condom, still in its branded wrapping, which somewhat spoils the “double blind” study pretence). Immediately after the earth has moved (or even if the session ended disappointingly, with a lost erection or broken prototype), each of them studiously fills in a detailed questionnaire.
‘I’s not the worst mood-killer we’ve encountered’: condom testers Christina Pederson and Brendan Weinhold. Photograph: Steve Schofield for the Guardian
Brendan and Christina had been married for 10 years when they started testing, and had seldom used condoms. Christina had only ever been with Brendan. And, to my surprise, the other California condom testers I spoke to had also only had sex with each other. Could they really be the best judges?
Amy and Max Hurwitz met in college and signed up to a CFHC study in their first year of dating. “Getting paid to have sex? Um, yes!” says Amy, over coffee in their West Hollywood apartment. Married with no children, they are an attractive couple: Max resembles a wholesome Clark Kent; Amy, young with curly brown hair, does most of the talking – which is fitting, as she did most of the testing work: inserting the rings, wearing diaphragms, sporting the cumbersome female condom. “We always thought of it as a good deed,” she says. “We called it ‘sex for science’. And because it was science, we always followed the rules – we weren’t going to fuck up science!”
What has been their least favourite test? The female condom, by far. The best? The IUD. They disagree on an ultra-thin, polyurethane condom, which Max describes as “bag-like”. “It wasn’t terrible, it would have been an acceptable option,” he says. “But it had this weird texture.”
“See, I liked it,” interjects Amy, “because it was clear and I could actually see his penis.”
The couple have pooled the money they earn doing studies – $100 each per trial – into a savings account, earmarked for vacations. And there has been another unexpected bonus: normal sex has become even more thrilling. “You have to wait three days after unprotected sex to have condom trial sex – so we found ourselves thinking, a lot of the time, ‘Are we going to have Study Sex, or Sex
Sex?’ It felt a little bit naughty.”
***
A 90-minute drive from Los Angeles, under bright studio lights, inventor and Gates Foundation grant winner Danny Resnic is working on a prototype that he hopes will revolutionise the condom. A solid grey, bullet-like phallus, ribbed vertically like a pleated skirt and nicknamed the Master, hangs above a mould, a white rectangular block of silicone with a circular hole at one end; the interior is a mirror image of the grey master.
“When I first said two decades ago that I was inventing new kinds of condoms, people asked: what could be different?” Resnic says. “Nobody was capable of imagining anything else. We are all so accustomed to one concept, we haven’t challenged it. But consumers are looking for something that is safe, and that actually addresses their need for pleasure.”
The reason we haven’t seen anything like this from the main condom manufacturers (Trojan, Durex, Skyn, Okamoto and Ansell, who account for 96% of the market), Resnic believes, is that the “oligopoly” has no incentive to change. They already control the market. “True change is going to have to come from outside the industry,” he says. “From the mavericks.”
There have been several over the years: Dutch inventor Jan Vinzenz Krause caused a stir when he announced a “spray-on” latex version in 2006, inspired by the mechanics of a car wash. (The main problem: it took five minutes to dry, which was a mood killer.) Last year the designers of the
Galactic Cap – an elfin latex hat that fits over the tip of the penis – raised more than $100,000 on Indiegogo, and plan to release it in 2017.
Resnic talks me through what he believes will be the condom of the future, an eccentric shape that has taken him more than 25 years to develop. Made of thick yellow latex, it resembles a squeaky dishwashing glove more than a condom. Yet, at this very moment, 28 South African couples – half heterosexual, half male homosexual – are road-testing his design. He’s testing it there, Resnic says, because the approval process was quicker and easier than in the US.
Danny Resnic with his vision of the future: the Origami Internal Condom, an eccentric shape it has taken him more than 25 years to develop. Photograph: Steve Schofield for the Guardian
Flaring out like a trumpet,
Resnic’s Origami Internal Condom comes in both “male” and “female” versions, and if it passes the grade, it will become the world’s first device explicitly approved for anal sex. Resnic is talking to me in the lounge of his LA condo on a bright morning. Tall, bald, with flawless straight white American teeth, wearing casual slacks and a collared red cotton shirt (this counts as business attire in LA), he pulls out a heavy black suitcase containing his prototype.
Using a veiny purple dildo, a translucent white Fleshlight (a masturbation toy about the size and shape of a torch, with a squishy interior) and a healthy amount of lubricant, Resnic illustrates how the condom would remain in place even with vigorous thrusting. When the superintendent of the building knocks on the door mid-demonstration, the familiar exchange suggests the super has seen this before.
A former art book publisher and interior designer, Resnic first conceived of a very ribbed super-strong condom 20 years ago. He had watched the love of his life and his best friends die of Aids in the 1980s, and had adopted diligent safe sex practices. But in 1993, a condom broke during sex and he, too, contracted HIV.
Condom breakage is rare: around four in 1,000 uses. Resnic was not just terrified, he was angry. How could he have used the best device available and still be handed what felt like a death sentence? “I had the idea for the Origami condom before I contracted HIV – but the diagnosis really gave me the motivation to pursue it,” he says. “I thought: there must be a better way to create safe sex.”
Twenty-five years and $2.5m later, he’s nearly there. “If I had known then what I do now, I’m not sure I would have seen the idea through to the end,” he admits. “I honestly thought it would take a couple of years when I started.”
But he couldn’t let go of the idea of a safe condom that enhances pleasure: a contraceptive that doubles as a sex toy – something that would make sex with a condom
better than sex without one. “Having sex with a traditional condom is like trying to taste a lollipop with
saran wrap on your tongue,” he says, handling a latex condom. “It’s just never going to feel amazing.”
Resnic went through many iterations. His idea was that the condom should have some movement, independent from the penis. His first models were folded like accordions, conjuring visions of sophisticated Japanese paper models.
An earlier Origami condom, with its male and female versions, was pleated horizontally rather than folded and made from silicone, designed to move up and down the penis (conventional latex condoms stay put). Tests found that 67% of couples liked using the Origami female condom, compared with just 16% for the female condoms on the market. The latest version of the Origami condom has thick lengthwise ribs, which Resnic says will be more pleasurable than traditional ribbed condoms (which are ribbed horizontally), as they push against the flesh. “The pleats create reciprocating motion by buckling and unbuckling,” he explains. “The fluting allows for tissue to move within these grooves. With the penis pushing against it, you get this yin and yang thing going on.”
Recently, he has made his most dramatic change: a switch from silicone to thick, opaque latex. Silicone turned out to be “too bulky”, and the latex condoms can be made by dip-moulding, which is much cheaper than injection moulding when it comes to mass production.
Will Resnic’s otherworldly, ribbed, buckling and gripping design prove the breakthrough that the Gates are looking for? Will men who dislike clothing their erections in thin sleeves of latex prefer thick ribbed chambers? Handling Resnic’s sturdy, opaque, beige sleeve – so thick it can stand upright – feels distinctly unerotic, but my hands are not the body part that the Origami condom will need to please. Sadly, Resnic won’t let me take a prototype home – I’m not taking part in a clinical trial, and this isn’t yet approved by the US Food and Drug Administration (FDA). But Resnic himself has used them, and assures me they do feel better than sex with a regular condom – and even better than sex without one.
***
Resnic has his detractors. He has been accused, in a series of articles on the rightwing Washington Free Beacon, of misspending his funding;
the site has claimed he is being investigated by the National Institutes of Health. According to the Beacon, a former employee of Resnic’s has provided them with proof that he spent his funding on plastic surgery, trips to
Costa Rica and the Playboy mansion, plus a condo in Provincetown. Resnic denies the allegations, and tells me the spending is due to a case of credit card fraud involving an ex-employee which, after a counter suit, he decided to drop. “The district attorney determined that the stolen money had all been spent by [the employee] and we decided to drop the expensive civil case when there was no chance to recover any funds,” Resnic says.
His design has its critics, too. “The Origami condom really is more of a sex toy that is inserted into somebody – as though you could stare your partner in the eyes while masturbating inside them,” says Mark McGlothlin, president of Apex Medical Technologies, whose lab is two and a half hours’ drive from Resnic’s, in San Diego. McGlothlin has worked in condom science for 35 years. “That doesn’t mean it’s bad sex,” he says of Resnic’s condom, “but it’s not the same thing. The point of a condom is to mimic natural intercourse.”
Apex is another Gates Foundation winner. His, like almost every other team, is trying not to reinvent the wheel, but to make it lighter and sleeker, with materials that are thinner but stronger than latex. While some are going hi-tech (University of Oregon chemists are working with polyurethane, University of Manchester scientists with graphene), Apex is going back to basics with collagen, giving animal intestines a makeover.
Lambskin condoms, fashioned from the intestines of slaughtered animals, have been used for centuries: the oldest sheep gut condom, recovered from a latrine in Dudley Castle in the West Midlands, was dated to around 1646. Today’s versions (such as
Naturalamb, available from chemists and online) are still fashioned from intestine, but sanitised with modern chemistry. They don’t account for a huge slice of the market and are used by those with latex allergies or who prefer the feel of genuine flesh over synthetic latex. McGlothlin thinks they are “disgusting”. “They stink like they came from an animal and they look like they came from an animal. Yet they still have wonderful properties.” The collagen in the skin, for instance, has far better heat transfer and water content properties than latex. People who use lambskin report that they do indeed feel more natural, but they are expensive, averaging £4 each, and – more damagingly – are considered too porous to protect against HIV.
Instead of using lambskin, McGlothlin’s proposal is to “upcycle” beef waste into reconstituted collagen. By grinding animal hide through a series of high-powered blenders and chemically treating the fluffy white fibres with a variety of plasticisers, surfactants and wetting agents, he is perfecting a condom that meets all the criteria: tactile, cheap and ready for the assembly line.
On paper, it sounds perfect: thoughtfully traditional yet hi-tech, and made of recycled materials. Unless you’re a vegetarian, is there anything else you could want from a condom? In Apex’s sunny San Diego lab, handling the prototypes, I could feel the creases in my fingers and tiny bumps on my skin through the condom. And it did not smell or look as if it came from an animal. Apex’s offices, housed in a sunny industrial park 25 miles from the Mexican border, look like those of any other business, stacked with full ring binders, photocopy machines, strip lighting and desks overflowing with paperwork. But the labs next door, where the tinkering takes place, house all kinds of condom gadgetry crafted to put new designs through their paces, from pumps used to inflate condoms with air to test their strength, to a phallic mould made of pure Teflon; there is a “Coital Model” that tests condoms with the highest degree of accuracy before they come into contact with real human genitals.
“We keep this under a cardboard box, because it might seem a bit obscene to our visitors,” says senior development engineer Scott Herrick, as he reveals a large and veiny plastic dildo on an air-powered pump, positioned across from a bright pink Fleshlight mounted on white plastic. Any experimental condom needs to undergo thousands of mechanical copulations before it’s considered safe enough for the real deal. Revved up, the simulator tests for strength. “How many thrusts do you think you get in one typical act?” Herrick says. A back-of-the envelope calculation: 300. “We want to be sure any prototypes we make are able to hold up and also to be able to compare them to other commercially available condoms. So we work with 500 thrusts, simulating a minimum of 13 minutes – somewhat of a guess, but probably not too far off.”

Any experimental condom needs to undergo thousands of mechanical copulations before it’s considered safe enough for the real deal. Photograph: Steve Schofield for the Guardian
It turns out that most condoms rip because the tip has worked its way down one side of the penis, until the pressure tears it. During the test I see, this condom remains resolutely in place as the coital replicator thrusts the dildo in and out of the Fleshlight, the air-powered pumps making an audible accompaniment, a bit like a steam engine. Herrick and I settle into a comfortable silence, enthralled by the spectacle.
***
After years of being drip-dried on to dildos, scrutinised with tweezers and plunged thousands of times into a mushy imitation vagina, nascent condoms enter the main event: the bedroom. More than 25,000 couples are registered with the CFHC to test condoms, penis caps, a huge variety of polymers, hormone implants, contraceptive rings, pills, gels and every other form of contraceptive weaponry in their arsenal.
“This is probably the weirdest thing we’ve worked with,” says Ron Frezieres, vice-president of research and evaluation at the CFHC. Standing on the fourth floor of an ordinary office block in downtown LA, he is holding up a pair of striped black knickers with an internally-fixed condom. The full-pelvis prophylactics – more chastity belt than sex aid – were designed by a Hawaiian woman who suffered from extreme herpes, ensuring any lover would not come into contact with her skin.
The CFHC is also developing its own new condom with Gates funding. Its design is a polyethylene condom, not stretchy and thick, but firm and thin. Technical description: “ultra sheer wrapping condom”. Or somewhat like clingfilm. “Our manufacturer has begged us, ‘Oh God, don’t compare it to saran wrap – people will think we’re making sandwich wrappers’,” Frezieres says.
Unlike most new designs – modelled on the old roll-on concept – polyethylene condoms are not taut and phallic, but baggy. A bit roomy, they truly are one-size-fits-all, clinging and wrapping instead of squeezing and constricting. They are very thin, about a quarter of the thickness of latex, and the polymer is less likely to degrade in hot climates, such as warehouses in India, Africa and South America, where latex condoms have been known to break down.
The donning method is easier: you roll it on like a sock using two pull tabs. “Zip and it’s on,” Frezieres says. No fumbling and fussing, no arousal-killing dithering – and a godsend if you live in an area without electric lights. Putting condoms on inside out (making them more likely to come off) is a serious issue in developing countries hit hardest by the HIV epidemic. Innovative, cheap and practical, is the CFHC design the Gates’s silver bullet?
“In all our years of research with condoms, we have learned that there is no silver bullet,” Frezieres says. “Some like them clear, others go for coloured, some prefer bumps, others like them ribbed. There’s so much variation in taste. Some even like flavoured condoms. I don’t know what the magic mix is, but I don’t think there is one single solution. The great challenge is to develop a bunch of new and creative ideas that push the entire field forwards.”
***
Pushing the field forwards sounds all well and good, but designs face several hurdles, from the sketchbook to the chemist, and not least the strict EU and US FDA safety regulations, some of which seem somewhat outdated. For example, a condom needs to be able to hold 16 litres of air before bursting. Apex’s collagen condoms fail this test. Is Herrick concerned that they won’t be what the FDA wants? “The FDA will only readily approve designs that meet the standards for latex condoms, which simply might not apply. Why should a non-stretchable condom have to hold 16 litres of air?”
The product also needs to be equivalent to another device already on the market. “Don’t invent something that will contradict the current standards – and it will be a decade before the standards change.” Resnic sighs. “In my opinion, making something that is similar to existing products isn’t the definition of innovation.”
One of Gates’s finalists isn’t redrawing the condom at all. “We decided not to use our money to develop our original condom design, but to do behavioural research in sub-Saharan Africa, to see what would really be practical for people in the places most hard-hit by HIV,” says Ben Strutt, head of design at the
Cambridge Design Partnership. “There are so many practical reasons why it’s not easy to just drop thousands of condoms into the region and expect the epidemic to go away,” he says, talking to me on the phone from his laboratory. “Women don’t carry handbags, for example, so how can you expect them to have condoms with them at all times? We were faced with the decision of carrying on with our narrow, technology-focused approach – or to create a solution that is more realistic. Sometimes people become too attached to their idea because it’s their baby, their sacred cow. Sometimes you need to look at the bigger picture, and put the sacred cow down.”

Glass moulds are dipped into a liquid beef compound, then dried, to make non-latex condoms. Photograph: Steve Schofield for the Guardian
The Cambridge team is looking at 18 different prophylactic concepts in order to create a “holistic condom solution”. They have brought on board a specialist in the Japanese affective design philosophy of kansei, or considering the emotional aspects of a product as well as the functional. “Whatever we create can’t just be functional – it has to be deeply sensory as well,” Strutt says. “It can’t be greasy, oily, thick, or something that destroys the moment.”
There have been some spectacular failures when it comes to this approach. A decade ago, there was an enthusiastic rush to create
microbicides: HIV-killing gels. The supposed holy grail of prophylactics, these would put women in control, and could be discreetly inserted like a thick lubricant. But
they proved ineffective, and in some trials actually worsened HIV infection rates. At the University of Manchester, the big idea is to use graphene, the super material composed of only the element carbon that won Manchester-based scientists Andre Geim and Kostantin Novoselov the
Nobel prize in physics in 2010. (Graphene is composed of the same element as diamonds and graphite, only arranged in a flat honeycomb lattice configuration.)
Dr Aravind Vijayaraghavan, a lecturer in nanomaterials, initiated a research group to apply for Gates funding to investigate embedding graphene into regular latex condoms. The idea is that it would allow them to be made much thinner (graphene is just one atom thick) as well as stronger. Adding graphene to condoms won’t jack up the price by more than 10 or 20%: “It’s not like we’re adding gold,” Vijayaraghavan says. But what the condom actually feels like or looks like, he will not divulge. A patent is pending.
Which of these new designs is most likely to disrupt the industry? William Potter is a British condom consultant who has overseen development in the R&D departments of major condom manufacturers since 1985. “There’s a huge amount going on at the moment,” he says, talking on the phone from his office in Cambridge. “But most of it is kept strictly confidential – the industry isn’t keen to publicise what it’s been doing because there is so much competition.”
The Gates initiative has galvanised awareness, he says, but there are many other schemes that get less attention. Personally, he is excited about one development on the horizon: a condom embedded with an anti erection-loss chemical, Zanifil. (This has been designed by a commercial manufacturer, for whom Potter is consulting.)
What does he make of the Gates Foundation’s mavericks? “The initiative has succeeded in bringing in new ideas and more speculative projects than the industry would have been capable of or willing to support. Whether or not that is a good idea, we will have to wait and see.”
***
Back in Glendale, southern California, I ask another condom-testing couple what they have learned from their years of homework. Tina and Don (not their real names) met in high school, have been together for 20 years and married for 10. The testing has been pretty good, they say, except for the female condom. “They are terrible and deserve their reputation,” Don says. “It’s like having sex with an artificial entity instead of a human being.”
Tina? “It makes loud rustling noises. It slips in and out. It leaves an unsightly baggy ring outside you. There is nothing erotic about it.”
But overall the trials have spiced things up. With jobs and two children, now aged five and seven, the couple found that sex was always falling to the bottom of their to-do list. After a day of work, picking the kids up from karate, cooking dinner and doing the laundry, intimacy was not a priority. Being guinea pigs – with “free birth control, the opportunity to contribute to science and get paid for it” – brought it back into the forefront of their minds.
“Sure, it took away from the spontaneity – but we had more sex overall,” Tina says. “And especially when the trials had deadlines.”
• Zoe Cormier is the author of Sex, Drugs And Rock’n’Roll, published by Profile Books at £12.99. To order a copy for £10.39, go to
bookshop.theguardian.com or call 0330 333 6846. Free UK p&p over £10, online orders only.